Ultrasound Elastography Systems in 2025: Transforming Diagnostic Imaging with Precision and Speed. Explore Market Growth, Breakthrough Technologies, and the Road Ahead.
- Executive Summary: Key Trends and Market Drivers in 2025
- Market Size and Growth Forecast (2025–2030): CAGR and Revenue Projections
- Technological Advancements: Shear Wave, Strain, and Beyond
- Competitive Landscape: Leading Manufacturers and New Entrants
- Clinical Applications: Oncology, Hepatology, and Musculoskeletal Uses
- Regulatory Environment and Standards (FDA, CE, and Global Bodies)
- Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
- Integration with AI and Digital Health Ecosystems
- Challenges: Reimbursement, Training, and Adoption Barriers
- Future Outlook: Innovations, Partnerships, and Strategic Opportunities
- Sources & References
Executive Summary: Key Trends and Market Drivers in 2025
The ultrasound elastography systems market is poised for significant growth in 2025, driven by technological advancements, expanding clinical applications, and increasing demand for non-invasive diagnostic tools. Elastography, which measures tissue stiffness to aid in the diagnosis of diseases such as liver fibrosis, cancer, and musculoskeletal disorders, is becoming an integral part of modern ultrasound platforms. The integration of elastography into conventional ultrasound systems is accelerating adoption across hospitals, diagnostic centers, and specialty clinics.
Key industry leaders such as GE HealthCare, Philips, Siemens Healthineers, and Canon Medical Systems continue to innovate, introducing systems with enhanced imaging quality, real-time quantitative analysis, and user-friendly interfaces. For example, Samsung Medison and Hitachi are also prominent in the sector, offering advanced shear wave and strain elastography solutions. These companies are focusing on expanding the clinical utility of elastography beyond hepatology, targeting oncology, cardiology, and women’s health.
In 2025, the market is witnessing a surge in demand due to the rising prevalence of chronic liver diseases and cancer worldwide. The World Health Organization continues to highlight liver disease as a major health burden, prompting healthcare systems to adopt more efficient, non-invasive diagnostic modalities. Ultrasound elastography’s ability to provide rapid, repeatable, and cost-effective assessments is a key driver for its increasing utilization.
Regulatory approvals and updated clinical guidelines are further supporting market expansion. The U.S. Food and Drug Administration and European regulatory bodies have cleared several new elastography-enabled ultrasound systems in recent years, facilitating broader clinical adoption. Additionally, professional societies are increasingly recommending elastography as a first-line or adjunctive tool for liver fibrosis staging and tumor characterization.
Looking ahead, the next few years are expected to see continued innovation, with artificial intelligence and machine learning being integrated into elastography platforms to enhance diagnostic accuracy and workflow efficiency. Companies are also investing in portable and point-of-care elastography devices, aiming to improve accessibility in both developed and emerging markets. As reimbursement policies evolve and clinical evidence grows, ultrasound elastography systems are set to become a standard component of diagnostic imaging, supporting earlier detection and improved management of a wide range of diseases.
Market Size and Growth Forecast (2025–2030): CAGR and Revenue Projections
The global market for ultrasound elastography systems is poised for robust growth from 2025 through 2030, driven by increasing clinical adoption, technological advancements, and expanding applications in both diagnostic and interventional medicine. As of 2025, the market is estimated to be valued in the low single-digit billions (USD), with projections indicating a compound annual growth rate (CAGR) in the range of 7% to 10% over the next five years. This growth trajectory is underpinned by rising demand for non-invasive diagnostic modalities, particularly in oncology, hepatology, and musculoskeletal imaging.
Key industry leaders such as GE HealthCare, Siemens Healthineers, Philips, and Canon Medical Systems continue to invest in research and development, introducing new elastography platforms and software upgrades that enhance image quality, workflow efficiency, and quantitative assessment capabilities. For example, Samsung Medison and Hitachi (now part of Fujifilm) have expanded their elastography portfolios, targeting both high-end and mid-range segments to address diverse clinical needs and budget constraints.
The market outlook is further strengthened by the growing prevalence of chronic liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and hepatitis, which are major drivers for elastography adoption in hepatology. Additionally, the integration of artificial intelligence (AI) and machine learning algorithms into ultrasound elastography systems is expected to improve diagnostic accuracy and reproducibility, supporting broader clinical acceptance and reimbursement. Regulatory approvals and guideline endorsements in major markets—including the United States, Europe, and Asia-Pacific—are also anticipated to accelerate market penetration.
Emerging economies in Asia-Pacific and Latin America are projected to experience above-average growth rates, fueled by healthcare infrastructure development and increasing awareness of early disease detection. Meanwhile, established markets in North America and Western Europe will continue to account for a significant share of global revenues, supported by ongoing investments in hospital and outpatient imaging facilities.
Overall, the ultrasound elastography systems market is set for sustained expansion through 2030, with leading manufacturers such as GE HealthCare, Siemens Healthineers, Philips, Canon Medical Systems, Samsung Medison, and Fujifilm expected to maintain their competitive positions through continuous innovation and strategic partnerships.
Technological Advancements: Shear Wave, Strain, and Beyond
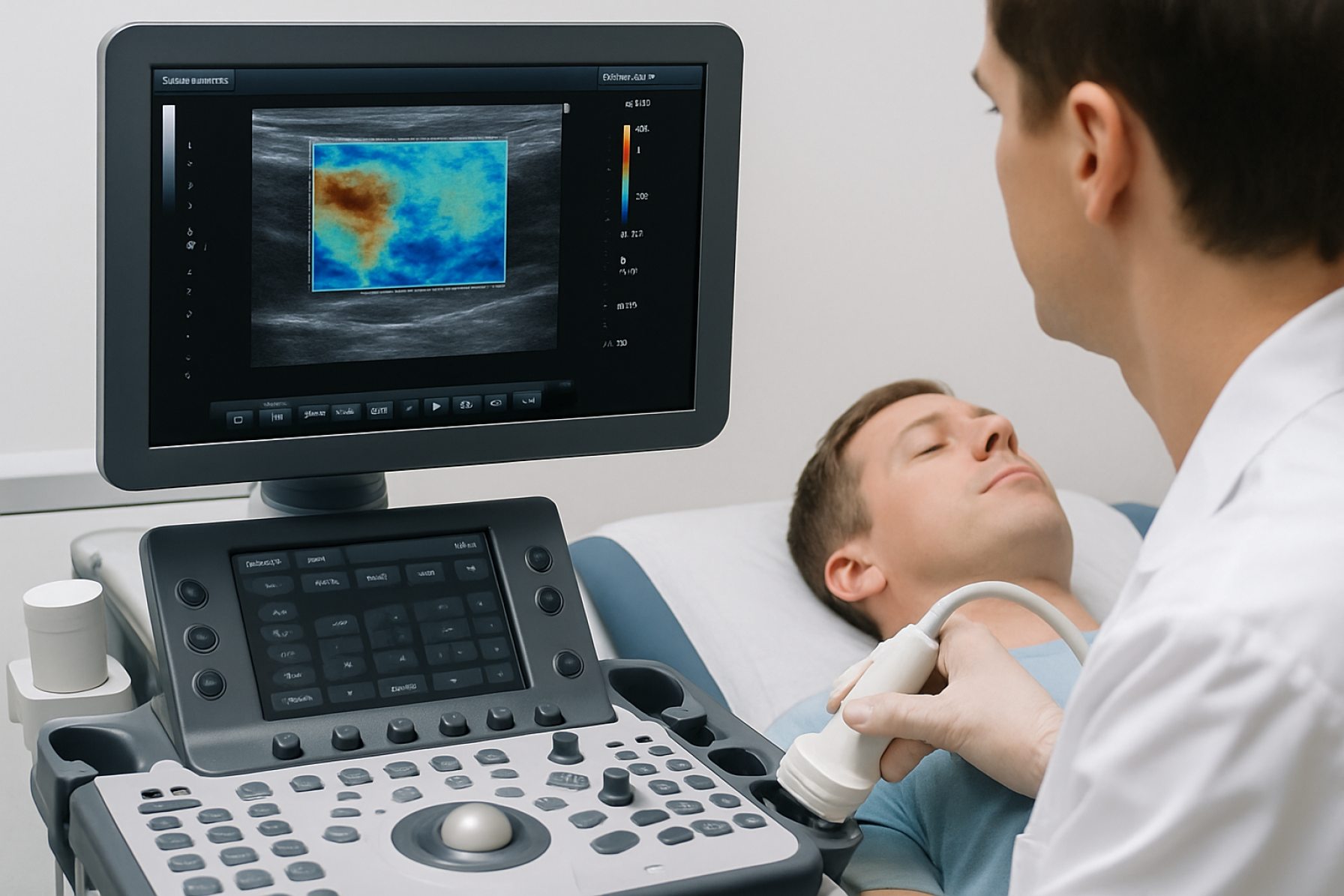
Ultrasound elastography systems have undergone significant technological advancements in recent years, with 2025 marking a period of rapid innovation and clinical adoption. The two principal modalities—shear wave elastography (SWE) and strain elastography—continue to evolve, driven by improvements in hardware, software algorithms, and integration with artificial intelligence (AI).
Shear wave elastography, which quantifies tissue stiffness by measuring the propagation speed of mechanically induced shear waves, has become a cornerstone in liver fibrosis assessment, breast lesion characterization, and musculoskeletal imaging. Leading manufacturers such as GE HealthCare, Philips, Siemens Healthineers, and Canon Medical Systems have all introduced next-generation SWE platforms. These systems now offer higher frame rates, improved spatial resolution, and real-time quantitative mapping, enabling more precise and reproducible measurements. For example, Siemens Healthineers has integrated advanced SWE into its ACUSON series, while Philips continues to expand elastography capabilities across its EPIQ and Affiniti ultrasound lines.
Strain elastography, which estimates tissue deformation in response to manual or physiological compression, remains valuable for applications such as thyroid, prostate, and breast imaging. Recent systems from Hitachi and Samsung Medison have enhanced strain imaging with automated quantification tools and improved user interfaces, reducing operator dependency and increasing diagnostic confidence.
A notable trend in 2025 is the convergence of elastography with AI-driven image analysis. Companies like GE HealthCare and Philips are incorporating machine learning algorithms to assist in lesion detection, segmentation, and risk stratification, aiming to standardize interpretation and reduce inter-observer variability. Additionally, portable and point-of-care elastography devices are gaining traction, with compact systems from Fujifilm and Mindray making elastography more accessible in outpatient and remote settings.
Looking ahead, the next few years are expected to bring further miniaturization, enhanced multimodal imaging (combining elastography with contrast-enhanced ultrasound or 3D imaging), and expanded clinical indications, particularly in oncology and chronic disease management. As regulatory approvals broaden and reimbursement pathways solidify, ultrasound elastography is poised to become an even more integral part of routine diagnostic workflows worldwide.
Competitive Landscape: Leading Manufacturers and New Entrants
The competitive landscape for ultrasound elastography systems in 2025 is characterized by a mix of established global manufacturers and a growing cohort of innovative new entrants. The sector is driven by rapid technological advancements, increasing clinical adoption, and expanding applications in oncology, hepatology, and musculoskeletal imaging.
Among the leading manufacturers, GE HealthCare maintains a prominent position with its LOGIQ and Vivid series, which integrate advanced shear wave and strain elastography capabilities. The company continues to invest in AI-powered image analysis and workflow automation, aiming to enhance diagnostic accuracy and user experience. Siemens Healthineers is another major player, offering the ACUSON Sequoia and S2000 platforms, which are widely recognized for their real-time elastography and quantitative tissue characterization features. Siemens is focusing on expanding elastography’s role in liver disease assessment and oncology, supported by ongoing clinical collaborations.
Philips has strengthened its elastography portfolio with the EPIQ and Affiniti ultrasound systems, emphasizing versatility and integration with advanced imaging modalities. The company is leveraging its global distribution network and partnerships with academic centers to accelerate adoption in both developed and emerging markets. Canon Medical Systems continues to innovate with its Aplio series, which features unique elastography modes such as Smart Elastography and Shear Wave Quantification, targeting both routine and specialized clinical workflows.
In Asia, Mindray and SonoScape are rapidly expanding their international presence. Mindray’s Resona and DC series offer competitive elastography solutions at accessible price points, appealing to a broad range of healthcare providers. SonoScape is gaining traction with its S and P series, which integrate elastography into compact and portable platforms, supporting point-of-care and resource-limited settings.
The competitive landscape is further energized by new entrants and specialized firms. Companies such as SuperSonic Imagine (now part of Hologic) have pioneered real-time shear wave elastography, and their Aixplorer system remains a reference in quantitative liver and breast imaging. Startups and smaller manufacturers are focusing on miniaturization, AI-driven automation, and cloud-based analytics, aiming to differentiate through cost-effectiveness and workflow integration.
Looking ahead, the market is expected to see intensified competition as regulatory approvals for new elastography applications accelerate, and as reimbursement policies evolve to support broader clinical use. Strategic partnerships, mergers, and acquisitions are likely, as established players seek to integrate novel technologies and expand their global reach. The next few years will be marked by a convergence of innovation, accessibility, and clinical validation, shaping a dynamic and competitive environment for ultrasound elastography systems.
Clinical Applications: Oncology, Hepatology, and Musculoskeletal Uses
Ultrasound elastography systems have become increasingly integral to clinical practice, particularly in the fields of oncology, hepatology, and musculoskeletal medicine. As of 2025, these systems are widely adopted for their ability to provide non-invasive, real-time assessment of tissue stiffness, which is a critical biomarker in various disease processes.
In oncology, elastography is now routinely used to characterize tumors and guide biopsies. The technology enables differentiation between benign and malignant lesions based on tissue elasticity, especially in breast, thyroid, and prostate cancers. Leading manufacturers such as GE HealthCare, Siemens Healthineers, and Canon Medical Systems have integrated advanced shear wave and strain elastography into their ultrasound platforms, allowing clinicians to assess tumor margins and monitor response to therapy with greater precision. For example, Samsung Medison offers systems with real-time elastography for breast and liver applications, supporting improved diagnostic confidence.
Hepatology remains one of the most prominent areas for elastography adoption. The non-invasive quantification of liver stiffness is now a standard of care for evaluating fibrosis and cirrhosis, reducing the need for invasive biopsies. Systems from Echosens—notably the FibroScan series—are widely used for liver assessment, while major ultrasound vendors have incorporated elastography modules into their general imaging platforms. The World Health Organization and leading hepatology societies continue to endorse elastography for chronic liver disease management, and ongoing improvements in software algorithms are expected to further enhance accuracy and reproducibility in the coming years.
In musculoskeletal medicine, elastography is increasingly utilized to evaluate tendons, muscles, and ligaments for injury, inflammation, and degeneration. The ability to visualize and quantify tissue stiffness aids in the diagnosis of conditions such as tendinopathy and muscle tears, and supports monitoring of rehabilitation progress. Companies like Philips and Hitachi have developed dedicated musculoskeletal elastography protocols, and ongoing research is expanding its use in sports medicine and rheumatology.
Looking ahead, the next few years are expected to bring further integration of artificial intelligence and machine learning into elastography systems, enabling automated quantification and improved workflow. The continued expansion of clinical indications, combined with growing evidence supporting elastography’s diagnostic and prognostic value, positions these systems as essential tools across multiple specialties.
Regulatory Environment and Standards (FDA, CE, and Global Bodies)
The regulatory environment for ultrasound elastography systems is evolving rapidly as these technologies become increasingly integral to diagnostic imaging worldwide. In 2025, the United States Food and Drug Administration (FDA) continues to play a central role in the approval and oversight of these devices. Most ultrasound elastography systems are classified as Class II medical devices, requiring 510(k) premarket notification to demonstrate substantial equivalence to legally marketed predicate devices. The FDA’s focus remains on ensuring device safety, efficacy, and quality, with particular attention to software validation, clinical performance data, and cybersecurity for connected systems. Major manufacturers such as GE HealthCare, Siemens Healthineers, and Philips routinely navigate these regulatory pathways for their elastography-enabled ultrasound platforms.
In the European Union, the transition from the Medical Devices Directive (MDD) to the Medical Device Regulation (MDR) has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. CE marking under MDR is now mandatory for all new ultrasound elastography systems, with manufacturers required to provide robust clinical data and comply with enhanced vigilance and reporting obligations. Companies such as Canon Medical Systems and Hitachi have adapted their regulatory strategies to meet these new standards, ensuring continued market access across Europe.
Globally, regulatory harmonization efforts are ongoing, with organizations like the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO) updating standards relevant to ultrasound elastography. The IEC 60601 series, which addresses the safety and essential performance of medical electrical equipment, and ISO 13485, which governs quality management systems for medical devices, are widely adopted benchmarks. In Asia, countries such as China and Japan have strengthened their regulatory frameworks, requiring local clinical data and registration for imported elastography systems. Leading Asian manufacturers, including Mindray</a), are actively engaging with these evolving requirements.
Looking ahead, the regulatory landscape is expected to become even more rigorous, with increased emphasis on real-world evidence, artificial intelligence integration, and interoperability standards. Regulatory bodies are also focusing on the standardization of elastography measurement protocols to ensure consistency and reliability across devices. As ultrasound elastography systems expand into new clinical applications, ongoing collaboration between manufacturers, regulators, and standards organizations will be critical to maintaining patient safety and fostering innovation.
Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
The global market for ultrasound elastography systems is experiencing dynamic growth, with regional trends shaped by healthcare infrastructure, regulatory environments, and adoption of advanced imaging technologies. In 2025 and the coming years, North America, Europe, Asia-Pacific, and emerging markets are expected to demonstrate distinct trajectories in the deployment and utilization of these systems.
North America remains a leading region, driven by high healthcare expenditure, robust reimbursement frameworks, and early adoption of innovative diagnostic tools. The United States, in particular, benefits from the presence of major manufacturers such as GE HealthCare, Siemens Healthineers, and Philips, all of which have established strong portfolios in elastography. The region is witnessing increased clinical integration of shear wave and strain elastography, especially in liver disease assessment and oncology. Ongoing FDA clearances for new elastography applications and systems are expected to further accelerate adoption through 2025.
Europe is characterized by a well-established public healthcare system and a strong focus on early disease detection. Countries such as Germany, France, and the UK are at the forefront, supported by the presence of key players like Echosens—a pioneer in transient elastography—and Hitachi, which has a significant footprint in ultrasound imaging. The European market is also benefiting from harmonized regulatory pathways and increasing investments in non-invasive diagnostic technologies. The adoption of elastography is expanding beyond hepatology into musculoskeletal and breast imaging, with continued growth anticipated as clinical guidelines evolve.
Asia-Pacific is projected to be the fastest-growing region, fueled by rising healthcare investments, expanding access to advanced diagnostics, and a large patient population with liver and metabolic diseases. Countries such as China, Japan, and South Korea are witnessing rapid uptake, with local manufacturers like Mindray and Fujifilm increasing their market share alongside global leaders. Government initiatives to improve early cancer detection and chronic disease management are expected to drive further demand for elastography systems in the region through 2025 and beyond.
Emerging markets in Latin America, the Middle East, and Africa are gradually adopting ultrasound elastography, though at a slower pace due to budget constraints and limited infrastructure. However, increasing awareness of non-invasive diagnostics and efforts by manufacturers to introduce cost-effective solutions are expected to support steady growth. Partnerships between global companies and local distributors are also facilitating technology transfer and training, laying the groundwork for broader adoption in the coming years.
Integration with AI and Digital Health Ecosystems
The integration of artificial intelligence (AI) and digital health ecosystems with ultrasound elastography systems is accelerating in 2025, driven by the need for enhanced diagnostic accuracy, workflow efficiency, and interoperability across healthcare platforms. Leading manufacturers are embedding advanced AI algorithms into their elastography platforms to automate image analysis, quantify tissue stiffness, and assist clinicians in detecting subtle pathological changes that may be missed by the human eye.
Major industry players such as GE HealthCare, Philips, Siemens Healthineers, and Canon Medical Systems are at the forefront of this transformation. These companies are leveraging AI to provide real-time decision support, reduce operator dependency, and standardize elastography measurements across different clinical settings. For example, AI-powered modules can now automatically delineate regions of interest, calculate liver stiffness scores, and flag suspicious lesions, streamlining the diagnostic process and supporting earlier intervention.
Interoperability with digital health ecosystems is another key trend. Ultrasound elastography systems are increasingly designed to integrate seamlessly with hospital information systems (HIS), electronic health records (EHR), and cloud-based data repositories. This connectivity enables remote consultations, telemedicine applications, and longitudinal patient monitoring, which are particularly valuable in managing chronic liver diseases and oncology cases. Companies like Samsung Medison and Mindray are actively developing platforms that support secure data sharing and AI-driven analytics within broader digital health frameworks.
In 2025, regulatory bodies are also adapting to these technological advances. There is a growing emphasis on validating AI algorithms for clinical use, ensuring transparency, and maintaining data privacy. Industry collaborations and partnerships with academic institutions are fostering the development of robust, clinically validated AI tools tailored for elastography applications.
Looking ahead, the next few years are expected to see further convergence of AI, cloud computing, and mobile health technologies with ultrasound elastography. This will likely result in more portable, user-friendly systems capable of delivering high-quality diagnostics in both traditional and remote care settings. As digital health ecosystems mature, elastography data will play an increasingly central role in precision medicine, population health management, and personalized treatment planning.
Challenges: Reimbursement, Training, and Adoption Barriers
Ultrasound elastography systems have demonstrated significant clinical value in non-invasive tissue characterization, particularly for liver fibrosis, breast lesions, and thyroid nodules. However, their broader adoption in 2025 and the coming years faces several persistent challenges, notably in reimbursement, operator training, and integration into routine clinical workflows.
Reimbursement remains a critical barrier, especially in regions where healthcare payers are slow to recognize the added value of elastography over conventional ultrasound. In the United States, the Centers for Medicare & Medicaid Services (CMS) have established reimbursement codes for certain elastography procedures, such as liver elastography, but coverage is not universal and often varies by state and payer. This inconsistency can deter healthcare providers from investing in advanced elastography-capable systems. Leading manufacturers, including GE HealthCare, Siemens Healthineers, and Philips, have been actively engaging with regulatory bodies and payers to expand reimbursement frameworks, but progress is incremental and region-dependent.
Operator training and standardization present another significant hurdle. Elastography techniques, such as shear wave and strain imaging, require specialized knowledge for both image acquisition and interpretation. Variability in operator skill can lead to inconsistent results, limiting clinical confidence and uptake. To address this, manufacturers like Canon Medical Systems and Mindray are investing in comprehensive training programs, including on-site workshops, e-learning modules, and simulation-based education. Additionally, industry bodies such as the Esaote and professional societies are working to establish standardized protocols and certification pathways, but widespread adoption of these standards is still evolving.
Integration into clinical workflows is also a challenge, particularly in busy radiology and hepatology departments. Elastography examinations can be time-consuming and may require additional steps compared to standard ultrasound. Workflow optimization, including seamless data transfer to electronic health records and automated reporting, is a focus for system developers. Companies like Samsung Medison and Hitachi are enhancing their platforms with user-friendly interfaces and AI-driven automation to reduce operator dependency and improve throughput.
Looking ahead, the outlook for overcoming these barriers is cautiously optimistic. As clinical evidence supporting elastography’s utility continues to grow, and as manufacturers and industry bodies collaborate on training and standardization, adoption rates are expected to rise. However, progress will likely remain uneven across regions, influenced by local reimbursement policies, healthcare infrastructure, and the pace of professional education.
Future Outlook: Innovations, Partnerships, and Strategic Opportunities
The future of ultrasound elastography systems in 2025 and the coming years is poised for significant transformation, driven by rapid technological innovation, strategic partnerships, and expanding clinical applications. As healthcare providers increasingly prioritize non-invasive diagnostic tools, elastography is emerging as a critical modality for assessing tissue stiffness in liver disease, oncology, musculoskeletal disorders, and beyond.
Major industry players are intensifying their research and development efforts to enhance the accuracy, speed, and versatility of elastography systems. GE HealthCare continues to invest in advanced ultrasound platforms, integrating artificial intelligence (AI) and machine learning algorithms to automate image interpretation and improve diagnostic confidence. Similarly, Philips is focusing on real-time elastography solutions that offer improved workflow efficiency and user experience, with a particular emphasis on liver and breast imaging.
Strategic collaborations are shaping the competitive landscape. For example, Siemens Healthineers is leveraging partnerships with academic institutions and clinical networks to validate new elastography techniques and expand their clinical utility. Meanwhile, Canon Medical Systems is working closely with healthcare providers to tailor elastography solutions for specific regional needs, particularly in Asia-Pacific markets where liver disease prevalence is high.
The integration of elastography into point-of-care and portable ultrasound devices is another key trend. Companies such as Samsung Medison and Mindray are developing compact systems that bring advanced elastography capabilities to outpatient clinics and remote settings, broadening access to early disease detection and monitoring.
Looking ahead, the next few years are expected to see further convergence of elastography with digital health platforms. Cloud-based data sharing, remote consultation, and AI-driven analytics are likely to become standard features, enabling more personalized and efficient patient care. Regulatory approvals for new elastography applications, such as thyroid and prostate assessment, are anticipated to expand the addressable market.
In summary, the outlook for ultrasound elastography systems is robust, with innovation and collaboration at the forefront. As leading manufacturers and healthcare organizations continue to invest in this technology, elastography is set to play an increasingly central role in precision diagnostics and value-based care worldwide.